Updated Sep 29, 2022
Immunoglobulins are protein molecules conferring antibody activity produced by terminal cells of B-cell differentiation known as plasma cells. Together, the B-cell immune lineage and their end-products, the immunoglobulins, are referred to as the humoral immune system to differentiate them from T cell-mediated immunity. There are five main classes of immunoglobulin (Ig) and they are found in different proportion in serum: 80% of serum immunoglobulin is IgG; IgM and IgA both make up about 5% each, 0.2% is IgD and only trace amounts of IgE are found in serum. Quantitative serum immunoglobulin tests assess the levels of the three major classes for cases of excess, reflecting chronic inflammation such as autoimmune conditions and B-cell malignancy such as myeloma, lymphoma, or chronic myeloid leukaemia (CLL), or instances of lack, so-called hypogammaglobulinemia, that reflects immunodeficiency and risk for infection.
What is globulin?
Globulin is a generic term for some sixty plasma proteins quantifiable through electrophoresis. They are globular proteins that form colloidal suspension (“jelly”) in blood, as distinct from most structural (fibrous) and membrane proteins. Although part of serum globulins, albumins, for instance, are more readily soluble in water. “Globulin” includes the antibodies (immunoglobins) and the glycoproteins.
Passing an electric current across filter-paper impregnated with blood allows globulin classification, from lightest to heaviest, into four basic serum gel-electrophoretic (EPG) bands:
- alpha-1 globulins
- alpha-2 globulins
- beta globulin
- gamma globulin (or immunoglobins)
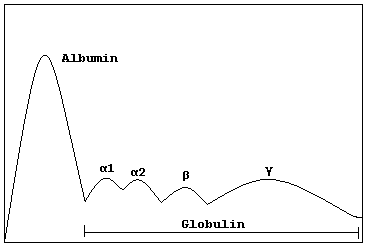 |
| Schematic representation of serum protein electrophoresis (serum EPG) [Wikimedia Commons] |
Gamma globulins are considered synonymous with immunoglobulins.
Back up a moment. Consider, for instance, were globulins fit in the spectrum of all proteins found in whole blood.
Centrifugation of whole blood separates it into its component plasma, thin buffy white-cell layer, and sizeable erythrocyte layer.
 |
| End result after blood centrifugation [Wikimedia Commons] |
- Solutions: components < 1 nm size – readily dissolved in water
- electrolytes
- gases
- glucose
- colloids: components 1 nm to 100 nm size – plasma proteins
- albumins
- globulins
- fibrinogen
- suspensions: components > 100 nm size – cellular components of whole blood
- red blood cells
- white blood cells
- platelets
The constituent human plasma proteins are then classified as follows:
 |
| Blood proteins showing the immunoglobulin fractions [Wikimedia Commons] |
Serum is essentially blood plasma without the fibrinogen—i.e. the supernatant after blood has been left to clot rather than spun—leaving only albumin and globulin. Serum contains more than 100 individual types of proteins. Proteins in serum can be fractionated according to electrical charge and size.
Soak filter paper in serum and pass an electrical charge across it. Densitometric scanning reveals the following trace in the paper (a) which can be converted into graph form (b):
 |
| With the exception of albumin, each fraction on the serum EPG contains more than one component |
Normal EPG |
| Normal serum protein electrophoretogram [Wikimedia Commons] |
Although albumin represents some 60% of the total protein in serum and consequently is responsible for the largest peak in the normal EPG, the globulins, which comprise most of the remaining fraction nevertheless represent the main focus of interpretation of serum protein electrophoresis.
EPG showing paraproteinaemia (e.g. Multiple Myeloma) |
| Serum protein electrophoretogram demonstrating a paraprotein [Wikimedia Commons] |
“Globulins” are a historical grouping of certain blood proteins of similar form—i.e. those proteins that form globules in water. By segregating serum proteins through electrophoresis, they can also be sub-classified according to size.

Functionally, then, globulins are a very diverse group: many acting as enzymes, others as messenger hormones (e.g. insulin), others as transporters of other molecules through cell membranes, others simply as amino-acid stock, still others that perform regulatory roles, and, in some cases, even structural proteins (e.g. actin and tubulin, which are globular and soluble as monomers, but polymerize to form long, stiff fibers).
- Alpha-1 globulins, for instance, include thyroid binding globulin (TBG), transcortin, glycoprotein and lipoprotein, and antitrypsin.
- Alpha-2 globulins include haptoglobin, glycoprotein, macroglobulin, and caeruloplasmin.
- Transferrin, some lipoproteins and some glycoproteins belong to the beta globulins.
- And, of course, the gamma globulins are almost exclusively made up of the immunoglobins.
The serum globulin level is reported in a comprehensive metabolic panel (CMP) of laboratory tests. The differential diagnosis of abnormal globulin levels is broad and, as such, an abnormal level is only the beginning of the search for a cause, the first steps of which are usually serum and urinary EPG, and not the end of the search:
Low Globulin levels
- Renal disease
- hepatic dysfunction
- celiac disease
- inflammatory bowel disease (IBD)
- acute hemolytic anemia
- agammaglobulinemia
- hypogammaglobulinemia
- Leukemia or other bone marrow disorders
- Autoimmunity diseases such as Lupus or collagen diseases
- Chronic inflammatory diseases such as Syphilis
- Waldenstrom’s macroglobulinemia
- Liver disease
- Rheumatoid arthritis
- Ulcerative colitis
- Carcinoid syndrome
- Kidney
- chronic viral or bacterial infection
A proper globulin to albumin ratio is 1:2, with a normal range between 1.7 and 2.2.
Certain disease processes are associated with abnormal amounts of gamma globulin in particular. This is called a gammopathy—though the term is generally reserved for an elevated gamma globulin (hypergammaglobulinemia) such as in “Monoclonal Gammopathy of Undetermined Significance” (MGUS) rather than a reduction of gamma globulin (hypogammaglobulinemia).
A gammopathy, moreover, can be either polyclonal, with a broad rise in the gamma-globulin EPG fraction, or monoclonal, giving a sharp spike in the EPG:
 |
| Image: McGraw Hill Higher Education |
Multiple Myeloma, a malignant proliferation of monoclonal plasma cells, is the classic cause of a monoclonal gammopathy.
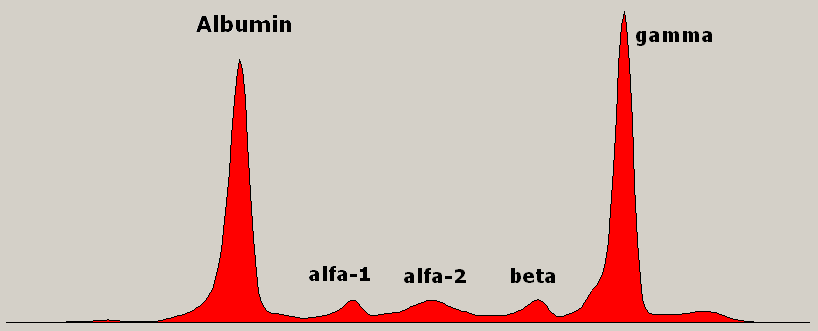 |
| The “monoclonal gammopathy” of Multiple Myeloma, seen as an abnormal spike on the EPG. [Wikimedia Commons] |
Apart from Multiple Myeloma, a monoclonal gammopathy can be seen in AIDS, Chronic Lymphocytic Leukaemia (CLL), Non Hodgkin’s Lymphoma (NHL), Hepatitis C, Systemic Lupus Erythematosus (SLE), and other connective tissue disorders, immunosuppression following organ transplantation, Waldesntrom macroglobulinemia, Guillain-Barre syndrome (GBS), and Tempi syndrome. The monoclonal protein may be the first discovery before a formal diagnosis of one of the above is made, hence monoclonal gammopathy’s (and hypergammaglobulinemia’s) primacy in medical parlance.
Summary: “Globulins” is a catch-all clinical term for the non-albumin plasma proteins and its importance comes from the various and serious disease processes that give rise to abnormal plasma globulin levels.
References:
- O’Connell TX, Horita TJ, Kasravi B. Understanding and interpreting serum protein electrophoresis. Am Fam Physician. 2005 Jan 1;71(1):105-12. PMID: 15663032.
Further Reading:
-
Buzzle. An Overview of the Serum Protein Electrophoresis (SPEP) Test. Available at http://www.buzzle.com/articles/an-overview-of-the-serum-protein-electrophoresis-spep-test.html as at 14th June 2016
- Bornhorst, J. American Academy of Clinical Chemistry (AACC). Protein Marker Evaluation of Monoclonal Gammopathies. Available at https://www.aacc.org/publications/cln/articles/2015/june/protein-marker-evaluation-of-monoclonal-gammopathies.aspx a at 14th June 2016
- The Best Practice Advocacy Centre New Zealand (BPAC). Making sense of serum protein bands. Available at http://www.bpac.org.nz/BT/2011/July/serum-protein.aspx as at 14th June 2016


