Pathways and Processes in Humans
Acid-Base
Acid is any molecule that can cleave off (Arrhenius) or donate (Brönsted) H+. Base is au contraire molecule that can cleave off OH– or accept H+. Acids and bases undergo metabolic conversion (e.g. lactate to glucose in gluconeogenesis, lactate to pyruvate and oxidation in cardiomyocytes), or excretion from the body. The human organism daily produces great quantities of acids – source of protons. These are volatile (respiratory) e.g. carbonic acid or non-volatile (metabolic) organic (lactic acid, fatty acid, ketone bodies, etc.) and inorganic acids (sulphuric acid, phosphoric acid, etc.).
The body maintains acid-base balance in the face of this acid load by various mechanisms:
- a chemical buffering system which deals instantaneously with pH changes
- a respiratory system reacts quickly (1-3 minutes) to eliminate carbonic acid through exhalation of carbon dioxide (hence, volatile)
- a more complex response over hour to days rendered by the kidneys
- detoxification of ammonium in the liver
- alteration in myocardial substrate utilisation






This approach leaves three parameters to assess:
- pCO2
- weak acids (mostly albumin)
- strong ion difference (SID)
Cellular Respiration – overview
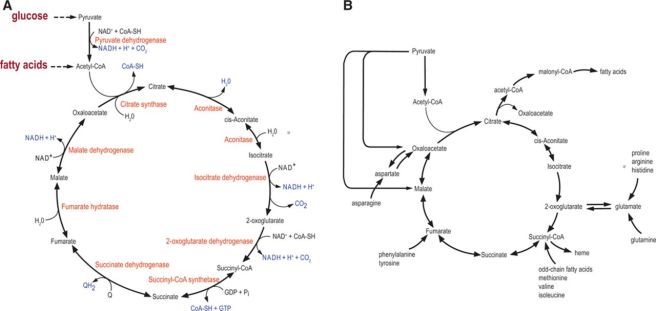
Acetyl-CoA stands at the crossroads of cellular metabolism:

Glucose metabolism
Glycolysis

Oxidation of Pyruvate

Krebs cycle
Oxidative Phosphorylation

Pentose Phosphate Pathway
The pentose phosphate pathway provides reduced NADPH and pentose sugars for nucleotide synthesis:

Protein Metabolism
Structure of amino acids

Peptide linkage

Instantiation of a polypeptide structure

Polypeptide (protein) folding


Heme metabolism
Heme-containing proteins
- Haemoglobin
- Myoglobin
- Cytochromes
- Catalase
- some peroxidases
Most hemoglobin degradation occurs in the macrophages of the spleen, where globin and iron are conserved for reuse:


Nucleosides and Uric Acid

Lipid Metabolism
Lipoproteins

Cholesterol metabolism
Cholesterol is primarily stored in the plasma membrane. Upon hormonal stimulation there is increased cholesterol absorption through the plasma membrane. When cholesterol is imported into the cell via the plasma membrane it greatly increases the cholesterol content stored elsewhere in the cell. (Rone, 2009).

Fatty acid metabolism

Steroid synthesis

References
- Berg JM, Tymoczko JL, Stryer L. “Chapter 22, Fatty Acid Metabolism.” Biochemistry. 5th edition. New York: W H Freeman; 2002. Available from: https://www.ncbi.nlm.nih.gov/books/NBK21173/
- Berg JM, Tymoczko JL, Stryer L. “Section 26.3, The Complex Regulation of Cholesterol Biosynthesis Takes Place at Several Levels.” Biochemistry. 5th edition. New York: W H Freeman; 2002. Section 26.3, The Complex Regulation of Cholesterol Biosynthesis Takes Place at Several Levels. Available from: https://www.ncbi.nlm.nih.gov/books/NBK22336/.
- Kaplan, L.J., Frangos, S. “Clinical review: Acid–base abnormalities in the intensive care unit.” Crit Care 9, 198 (2004). https://doi.org/10.1186/cc2912.
- Morgan, T John. “The Stewart approach–one clinician’s perspective.” The Clinical biochemist. Reviews vol. 30,2 (2009): 41-54.
- Rone, Malena B et al. “Cholesterol transport in steroid biosynthesis: role of protein-protein interactions and implications in disease states.” Biochimica et biophysica acta vol. 1791,7 (2009): 646-58. doi:10.1016/j.bbalip.2009.03.001.
